Amidst Rapidly Increasing COVID-19 Cases, Studies Indicate A Vaccine Is Closer than We Think
Photo courtesy BBC News
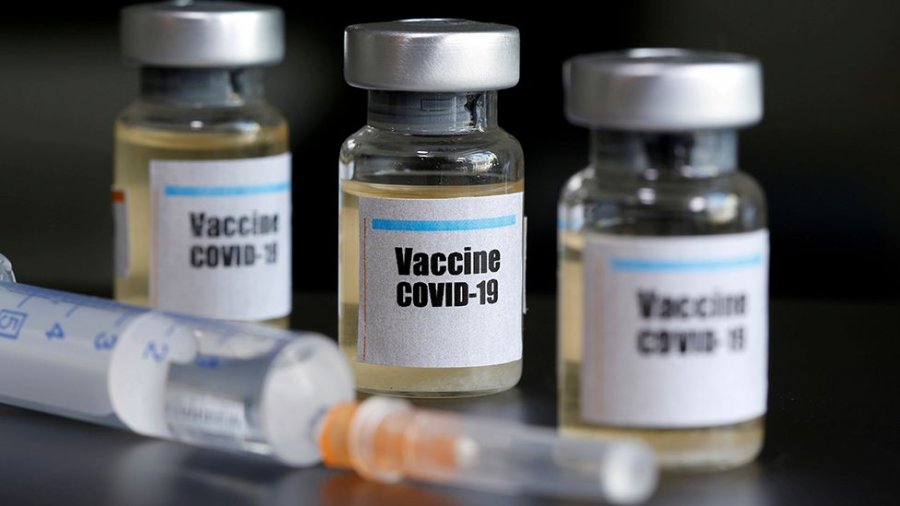
Despite a massive spike in COVID-19 cases nationwide, news of several breakthroughs in the vaccine-development-process may be a beacon of hope for the United States as well as the world at large. On Monday, November 9th, American pharmaceutical behemoth Pfizer, in tandem with German partner BioNTech, announced that data collected from their recent trials indicates 90% vaccine effectiveness. This statistic was later updated to 95%, following the release of further research. Just one week after Pfizer’s initial news—on Monday, November 16th—Massachusetts-based pharmaceutical manufacturer Moderna announced their vaccine had a 94.5% efficacy rate, and that they would begin vaccinating select groups as soon as January. They estimated that the general population will be vaccinated as soon as April. The implications of this discovery could be massive—a projected 1.3 billion doses of the Pfizer vaccine alone could be administered within the next year, granting temporary immunity to millions of essential and frontline workers, high-risk groups, and the overall worldwide population.
The pharmaceutical industry’s race against the virus has been tumultuous since the first case of coronavirus was confirmed in Wuhan, China last November. A multitude of corporations have taken various approaches to developing COVID-19 treatments and prophylactic remedies. Both Moderna’s and Pfizer’s vaccines employ an unconventional mechanism–messenger RNA–to try to create antibody responses in the vaccines’ recipients. The clinical trials for the Pfizer vaccine were conducted among a sample size of 43,000 volunteers, half of whom received a placebo vaccine. Among 94 confirmed cases of the virus, the vaccine’s success rate was calculated at approximately 90%, after the administration of a second dose. Pfizer claims that after 164 confirmed cases have been examined, they will advance past the data collection stage and begin mass-manufacturing. Moderna’s vaccine trials involved 30,000 people following the same placebo procedure. Out of the 15,000 vaccinated, only five developed COVID-19, which was symptomatically mild in all five cases. Side effects were reported as minimal.
The rollout process of vaccine administration will likely involve certain setbacks for the United States. Both vaccines are two-dose products, meaning that the number of manufactured doses will need to be halved when calculating the number of recipients. Given the rushed nature of the research, it is also too early to observe long-term ramifications of administering the vaccine to individuals. Various strains of the mutating virus will require frequently updated treatment. Finally, the Pfizer vaccine requires a dramatically lower refrigeration temperature than the Moderna vaccine, making it less accessible to more impoverished countries with fewer resources.
The American government has expended billions of dollars on private vaccine research and development alone, and it has officially announced that vaccines will be free for everyone. Essential workers, frontline healthcare providers, and high-risk individuals will receive the vaccine first, and then the general public will follow. Purportedly all groups will be vaccinated by the end of 2021 at the latest. If vaccination administration pans out the way it is predicted to, it is possible that we will regain a sense of semi-normalcy and experience an economic recovery sooner than we think.