COVID-19 Booster Shots Spark Controversy in Medical Community
Photo courtesy Times of Bristol
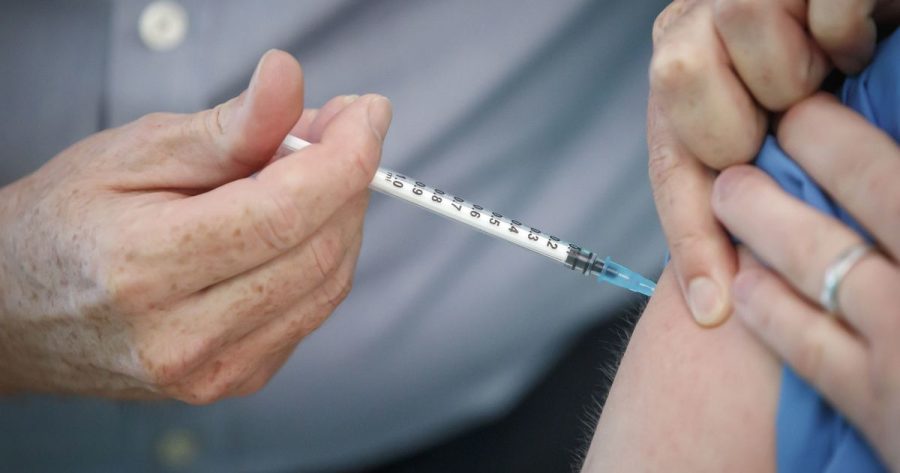
COVID-19 Booster Shot
As COVID-19 cases continue to rise, booster shots are becoming a topic of debate amongst scientific and healthcare communities. The release of coronavirus booster shots has been in the works even prior to the release of the vaccine itself, with Dr. Anthony Fauci’s support since January.
The first people administered booster doses are the same as for the initial vaccine: healthcare professionals, at risk citizens, those in long-term care facilities, and people with high coronavirus exposure. In addition to meeting these requirements, one can only receive a booster dose six or more months after their second shot. Third doses for immunodeficient people had already attained FDA approval for a few months. However these are considered additional shots rather than boosters, because they are used to reach a normal post-vaccination immunity level rather than increase them after they have faded over time.
The controversy surrounding booster shots is not about function, but rather necessity. While Dr. Fauci, who relied on studies by Israeli scientists, the National Institutes of Health (NIH), and the National Institute of Allergy and Infectious Diseases (NIAID), is highly supportive of boosters, many others question if additional doses are worthwhile. Research has shown that immunity against minor COVID-19 cases wanes over time, but various scientists, most notably Dr. Krause and Dr. Gruber from the FDA, point to studies that show vaccine efficacy against severe cases remains high. These results bring up the question of whether or not booster shots are a good use of vaccines.
Much of the world is still struggling to deliver vaccines, causing the World Health Organization to release a statement asking that countries with a surplus of vaccines hold off on administering additional shots until the end of the year. This way, each country can vaccinate at least 40% of its population. Others add that using the doses to immunize more people globally will reduce the chances of another, potentially deadlier variant emerging, and will further benefit all-around health, more so than using them on already vaccinated people.
The only available booster shot is Pfizer, meaning that those who received Moderna or Johnson & Johnson doses cannot immediately have access. Some are worried that only allowing one company’s vaccine will be difficult to implement, with it affecting a select group of vaccines rather than all of them. Meanwhile, there is concern that booster shots could imply that the vaccines are dysfunctional and unnecessary, and fuel anti-vax groups.
According to a Kaiser Family Foundation survey of 1,519 randomly selected adults, 71% of unvaccinated participants voted that the implementation of booster shots proves that the vaccine itself does not work. In contrast, 78% of vaccinated respondents said boosters prove scientists are working to find ways to make vaccines more effective.
On September 22, the FDA approved booster shots, and two days later the Centers for Disease Control (CDC) followed suit. With this authorization, though, the impact of booster shots remains to be seen.

Grade: 12
Years on Staff: 3
Why are you writing for the Flintridge Press?
There are very few opportunities to write outside of a classroom setting,...